The US Food & Drug Administration (FDA) today approved pretomanid for the treatment of highly drug-resistant forms of tuberculosis (TB) in a combination regimen known as BPaL. This regimen was developed by TB Alliance. Over the past year KNCV collaborated with TB Alliance on a study of planning needs and acceptability of this regimen in different countries. “This endorsement marks the beginning of a new era. KNCV stands ready to support countries to introduce and quickly scale up this life saving treatment,” says Kitty van Weezenbeek, executive director of KNCV.
TB is the most deadly infectious disease worldwide, taking 1.6 million lives every year. Unnecessary, because TB can be cured with the right medicines and treatment. For highly drug-resistant (multi drug-resistant and extensively drug-resistant) TB, however, this is a lot more difficult. The overall treatment success rate of extensively drug-resistant TB worldwide is only 34 percent. The first results of the BPaL regimen show a rate of 90 percent.
The BPaL regime consists of the drugs bedaquiline, pretomanid and linezolid. Pretomanid is a new drug against TB. The combination of these three oral medications means that highly drug-resistant TB patients no longer have to undergo painful injections for months. Moreover, treatment with BPaL ‘only’ takes 6 months, as opposed to the 18 to 24 months that is currently recommended for patients with highly drug-resistant TB.
Over the past few years KNCV already focused on country readiness for uptake of new drugs, introducing and scaling up the use of new drugs and regimens in many countries. This groundwork will facilitate early uptake of the BPaL regimen in these countries.
Van Weezenbeek: “BPaL will not only bring hope and cure for patients, but will also facilitate the delivery of care by individual health professionals and an effective response of health systems to patients with highly drug-resistant TB. KNCV recognizes the breakthrough that this regimen will bring. In addition to facilitating introduction and scaling up, it is important to generate evidence of its use under programmatic conditions; at the same time responsible use of pretomanid-based regimens needs to be ensured. After all, we have to protect our new medicines! We look forward to working with all stakeholders on enabling patient access to BPaL. No time to waste.”
More about the BPaL regimen on the website of TB Alliance.
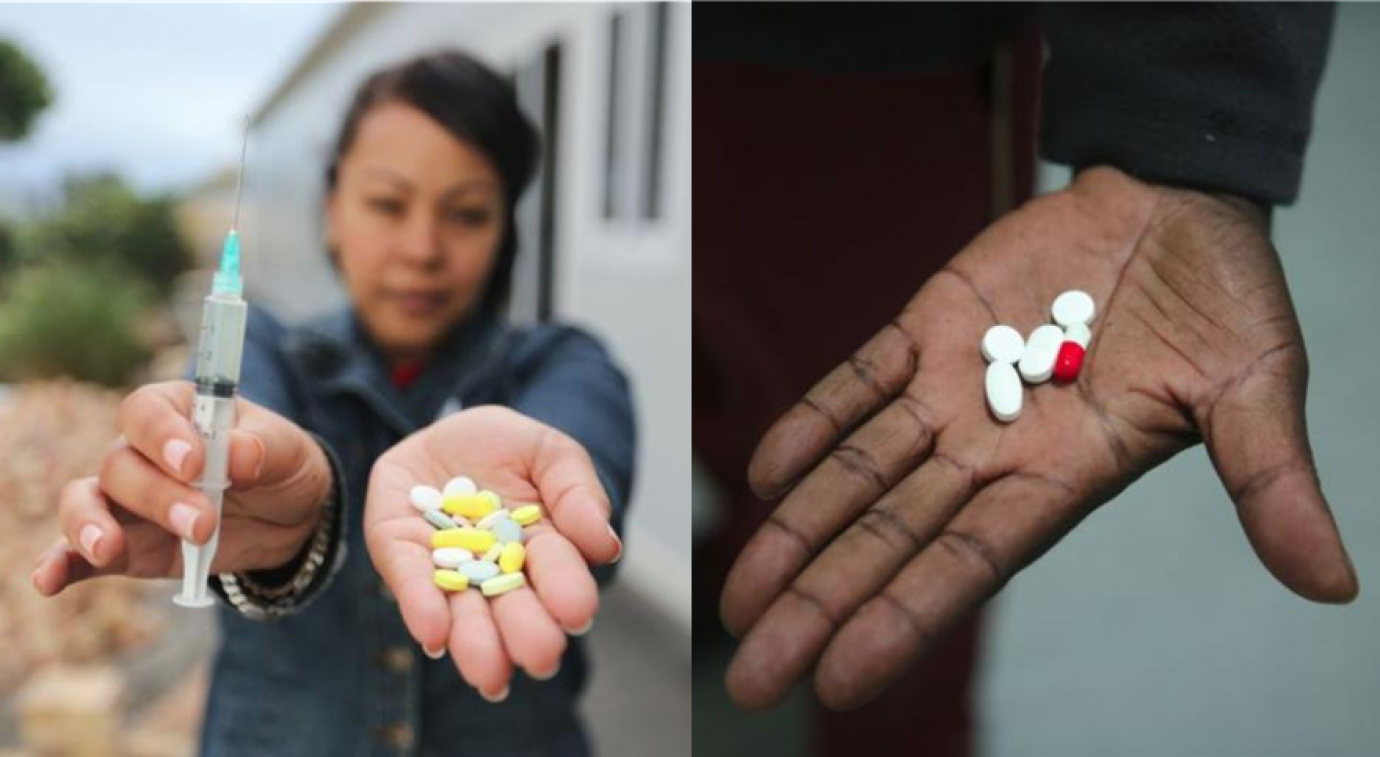
PHOTO: Current recommended treatment regimen for highly drug-resistant TB (left) versus the BPaL regimen (right).
PHOTOCREDIT: TB Alliance